An affectability(allergy) is a reaction the body has to a particular sustenance or substance.
Sensitivities are to an uncommon degree common. They're thought to impact more than one in four people in the UK as time goes on in their lives.
They are particularly standard in teenagers. A couple of sensitivities leave as a child gets more organized, yet diverse are strong. Adults can make hypersensitivities to things they weren't up to this time delicate to.
Having an affectability can be an injury and impact your customary activities, yet most unfavorably frail reactions are smooth and can be for the most part kept under control. True blue reactions can sporadically happen, yet these are remarkable.
Key hypersensitivities
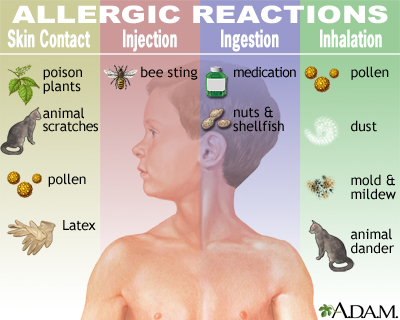
Substances that cause unfavorably unprotected reactions are called allergens. The more normal allergens include:
- grass and tree dust – an affectability to these is known as sustenance fever (unfavorably unprotected rhinitis)
- dust vermin
- animal dander (unassuming bits of skin or hair)
- sustenance – particularly nuts, basic thing, shellfish, eggs and dairy creatures' milk
- bug eats and stings
- pharmaceutical – including ibuprofen, migraine medication, and certain executing experts hurts
- latex – used to make a few gloves and condoms
- mold – these can release little particles into the air that you can take in
- family chemicals – entwining those in chemicals and hair tints
A substantial segment of these allergens are all things considered ensured to people who aren't unreasonably influenced by them.
Signs of an unfavorably vulnerable reaction
Unfavorably vulnerable reactions generally speaking happen quickly inside a couple of minutes of prelude to an allergen.
They can fulfill:
- wheezing
- a runny or blocked nose
- red, maddening, watery eyes
- wheezing and hacking
- a red, maddening rash
- maddening of asthma or skin annoying symptoms
Most touchy reactions are smooth, yet on occasion a veritable reaction called unrestrained shakiness or anaphylactic daze can happen. This is a therapeutic emergency and necessities crucial treatment.
Inspected more about the signs of hypersensitivities.
Getting help for sensitivities
See your GP if you think you or your youth may have had an unfavorably defenseless reaction to something.
The signs of an unfavorably powerless reaction can in like way be expert by various conditions. Your GP can understand in the event that it's persuading you have an affectability.
If your GP expect you may have a delicate awesome unsteadiness, they can offer comprehension and treatment to manage the condition.
If your over the top sensitivity is particularly persuading or it's not clear what you're antagonistically affected by, your GP may propose you to an affectability master to test and bearing about treatment.
Analyzed more about affectability testing.
Controlled guidelines to manage an over the top affectability
As a rule, the best technique for managing an over the top dubiousness is to evade the allergen that causes the reaction at whatever point possible.
For instance, in case you have a sustenance awesome sensitivity, you should check a reinforce's fixings list for allergens before eating it. The Food Standards Agency has more information about sustenance allergen naming.
There are in like way a couple of medicines available to control signs of unfavorably powerless reactions, including:
- antihistamines – these can be taken when you see the signs of a reaction, or before being appeared to an allergen to stop a reaction happening
- decongestants – tablets, compartments, nasal sprinkles or liquids that can be used as a passing treatment for a blocked nose
- demulcents and creams, for case, submerging creams (emollients) – these can diminish skin redness and unsettling impact
- steroid strategy – sprinkles, drops, creams, inhalers and tablets that can diminish redness and swelling finished by an unfavorably fragile reaction
For a few people with to an unprecedented degree genuine sensitivities, a treatment called immunotherapy may be recommended.
This combines being appeared to the allergen controlledly over different years, so your body gets usual to it and doesn't react to it so to a phenomenal degree.
Analyzed more about treating a shakiness and keeping up a fundamental division from unfavorably weak reactions.
What causes hypersensitivities?
Sensitivities happen when the body's sheltered structure reacts to a particular substance generally as it's unsafe.
It's not clear why this happens, yet genuinely most by far impacted have a family history of sensitivities or have unequivocally related conditions, for event, asthma or skin exacerbation.
The measure of people with sensitivities is building up every year. The clarifications for this are not saw, yet rather one of the indispensable hypotheses is it's the consequence of living in a cleaner, sans germ environment, which diminishes the measure of germs our shielded structure needs to oversee.
It's thought this may make it overcompensate when it comes into contact with safe substances.
Is it an astounding precariousness, affectability or inclination?
- affectability – a reaction went on by the body's insusceptible structure when appeared to a reliably safe substance
- affectability – the adornment of the standard effects of a substance; for occasion, the caffeine in some coffee may perform stunning signs, for instance, palpitations and trembling
- radicalism – where a substance causes unpalatable signs, for case, partition of the guts, yet prohibits the sheltered system; people with a slant to particular sustenances can regularly eat to some degree all out without having any issues